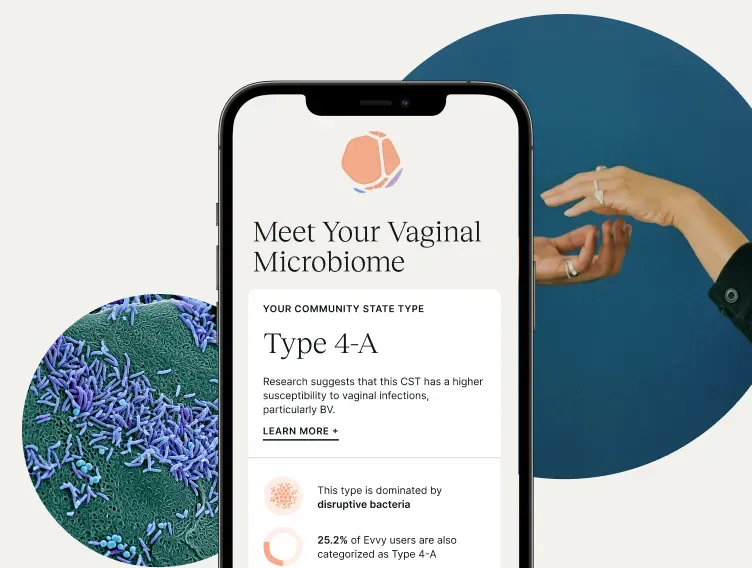
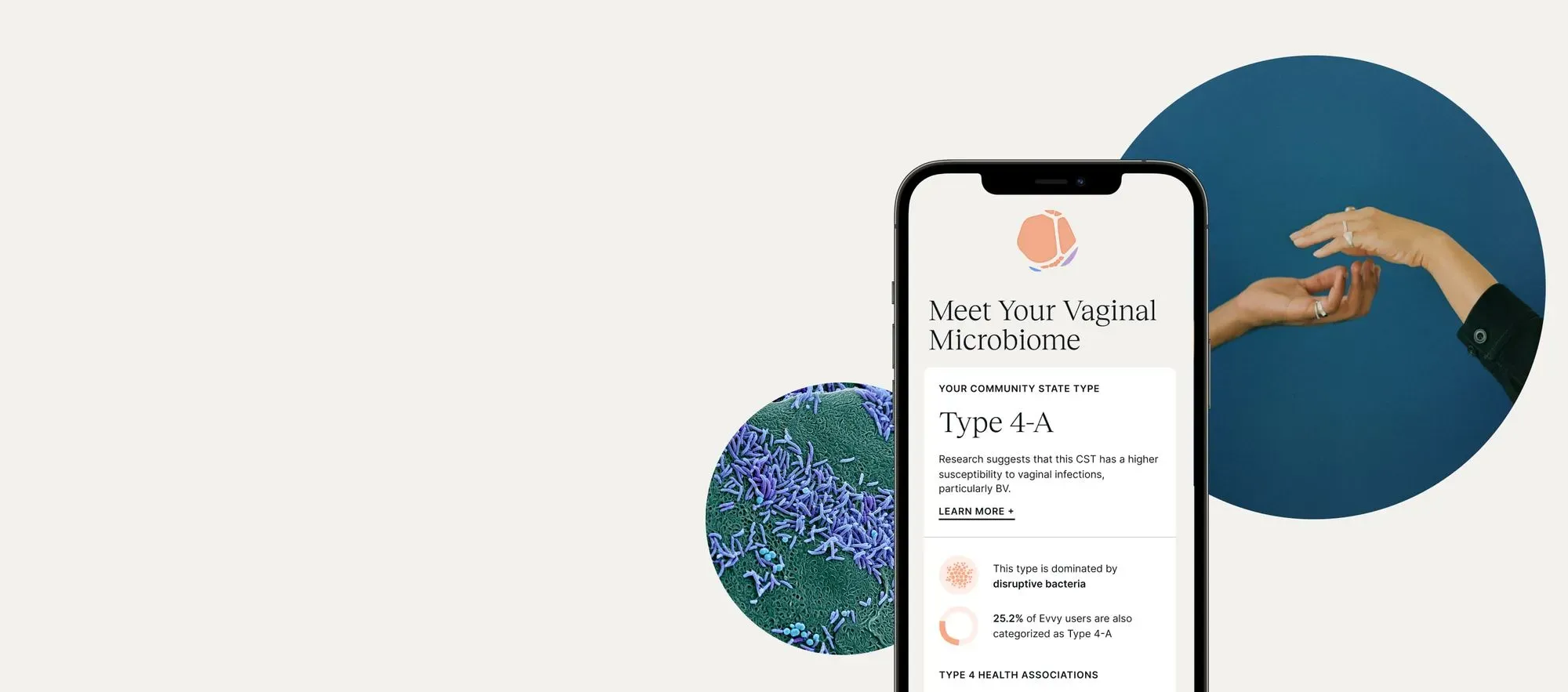
A Guide to Vaginal Testing Methods
What are the different vaginal testing types available, what can they tell you, & where do you get them? Learn the pros and cons of the methods used today.
Words by Dr. Krystal Thomas-White, PhD
Scientifically edited by Dr. Krystal Thomas-White, PhD
Medically reviewed by Dr. Christine Vo, MD
When you visit the doctor complaining about symptoms like itching, burning, and discharge, you hope to walk out with a clear picture of what’s causing them and some treatment that will give you relief.
However, diagnosing and treating vaginal health issues isn't always that easy. The current standard for diagnosing vaginal conditions often relies on vague data, and vaginal health testing often depends on patients to self-identify their symptoms because we lack the data or tests to reach specific diagnoses. Due to the subjective nature of the testing and the variation in testing methods, comparing results from different mechanisms can be confusing.
To help with this, we’ve put together a comprehensive overview of the different vaginal testing methods, along with their pros and cons. Next time you go to your doctor, ask them which method they use and then you can review our guide below to learn more about it!
(This guide goes through all vaginal testing methods, including both full vaginal microbiome tests and vaginal testing methods that are based on symptoms, microscopy, and other limited information. If you’re looking to explore just the differences between at-home vaginal microbiome tests, check out our guide here instead.)
1. Amsel criteria (symptoms-based)
Amsel criteria or symptom-based diagnostics are the most commonly performed test for bacterial vaginosis, a type of bacterial infection. This test is named after Dr. Amsel who, in 1983, described a set of criteria for diagnosing “nonspecific vaginitis” (or what we now know as bacterial vaginosis (BV).
How it works
If a patient comes into the doctor’s office with symptoms consistent with BV, the doctor can use the Amsel method to determine a BV diagnosis.The diagnosis is considered positive if a patient has three of the four criteria:
- Excessive vaginal discharge
- A vaginal pH greater than 4.5 (A healthy vagina typically has a vaginal pH level between 3.8 and 4.5, which helps maintain a balance of good bacteria.)
- A positive whiff test (This is when a clinician smells the vaginal discharge after adding potassium hydroxide. If they smell a fishy odor, then the sample has a positive whiff test.)
- Clue cells (based on a wet mount)
A vaginal wet mount test (also called a vaginal smear or wet prep) is when a doctor looks at a sample of vaginal discharge under the microscope. In this case, they are looking for “clue cells” — a vaginal epithelial cell (the cells that make up the vaginal wall) covered in bacteria. In the photo above, the larger blobs are epithelial cells, also known as squamous cells. The tiny pinpoint dots are bacteria. The clue cell is the large epithelial cell completely covered in bacteria.
What does a result look like?
The results of an Amsel test don’t come with lab results and instead are delivered with a verbal “positive/negative” from your doctor during your visit. With the Amsel test, your doctor will likely ask you about your symptoms, take a swab from your vagina, leave your exam room, and come back during the same visit to deliver the diagnosis.
When your doctor is “BRB,” they’re likely looking at your swab under the microscope to conclude whether or not you meet three of the four above Amsel criteria.
Pros
- Fast: can be done at the doctor’s office with immediate results.
Cons
- Unreliable: Not all women suspected of having bacterial vaginosis meet all of these criteria.
- Imprecise: The Amsel does not provide any information on the types of bacteria present which can make it difficult to provide the correct diagnosis and prescribe the most effective treatment, as different antibiotics work better on certain types of bacteria.
Requires equipment: Requires a doctor to have a microscope in their office and be trained on how to use it.
Recurrent symptoms? Get Evvy's at-home vaginal microbiome test, designed by leading OB-GYNs.
2. Nugent score (microscope-based)
Nugent tests are primarily used for scientific research and rarely used in clinical practice as a bacterial vaginosis test. Similar to the Amsel, a Nugent test also requires a doctor to look at a sample of vaginal discharge under a microscope, but in this process, a stain is added (called a Gram stain).
When the stain is added, bacteria either show up purple (Gram-positive) or pink (Gram-negative). The staining corresponds to how thick the cell wall is. A thicker cell wall = darker purple = Gram-positive (e.g. Lactobacillus), a thinner cell wall = lighter pink = Gram negative (e.g. E. coli). Some microbes, like Gardnerella, are small Gram variable cocci (can stain either purple or pink).
How it works
To do a Nugent test, a clinician must look at a stained sample under a microscope and count the number of different types of cells present. They then give the sample a score between 1-10. A score of 0-3 indicates “normal” lactobacilli, 4-6 intermediate, and 7-10 indicates high diversity and a positive bacterial vaginosis diagnosis.
Although the Nugent criteria is considered the gold standard by the World Health Organization (WHO), it only measures the physical diversity of bacteria within a sample and doesn’t classify the bacteria present.
However, it can be misleading because some organisms don’t stain as you would expect. For example in the figure below, L. iners (a) can sometimes end up looking more like Gardnerella (c) than they do other lactobacilli (b). Also, the problem with Nugent is that it is not a measurement of bacterial vaginosis, but a measurement of diversity. Not all women with high Nugent scores have symptoms.
What does a result look like?
Nugent tests are rarely performed at the doctor’s office and are mostly used for research so it’s unlikely that your doctor will use this method to diagnose you.
True to its name, the Nugent test will give a Nugent score based on your doctor’s interpretation of the Gram stain — with a lower score indicating lactobacilli dominance and a higher score indicating a diversity of bacteria. More specifically, the score ranges are: “normal” lactobacilli (as score from 0-3), intermediate (a score of 4-6), and bacterial vaginosis (a score of 7-10).
Pros
- Fast: Can be completed at the doctor’s office by anyone with training, reagents, and a microscope. Note that this method is rarely done in the doctor's office and few clinical labs provide it as a test. Instead, this is primarily used for research purposes.
Cons
- Unreliable: Given that the clinical definition of bacterial vaginosis requires a patient to have symptoms, the Nugent score can produce a result of “positive bacterial vaginosis” for people who have a diverse microbiome but no symptoms (and therefore would not have clinically diagnosed bacterial vaginosis).
- Imprecise: Doesn't identify the specific organisms present, making it difficult to provide the correct diagnosis and prescribe the most effective treatment.
- Misclassification: Key vaginal microbes look very similar under the microscope (even if they're very different in terms of their positive or negative implications), so they can be confused for each other when a diagnosis depends on microscopy.
- Requires equipment: Requires a doctor to have a microscope in their office and be trained on how to use it.
3. Culture
A culture can be considered the old-school method of detecting bacteria.
How it works
Culturing involves adding a sample of vaginal discharge to a nutrient-rich growth-media (the agar or broth the bacteria is grown in) and waiting for something to grow.
Unfortunately, not all bacteria grow well in cultures. Some fungi and bacteria (like Mycoplasma) are notoriously slow-growing or finicky, and it is hard to routinely grow them. This is because bacteria are like plants, and some need very specific conditions to grow. For this reason, microbial culture is generally limited to the “weeds” of the microbial world — the organisms that can grow anywhere no matter the conditions.
What does a result look like?
Very few clinics do routine vaginal culturing. It's time-consuming and many common vaginal pathogens, like Gardnerella and Prevotella, are hard to grow in the lab.
However, culturing for other urogenital disorders, like UTIs, is still quite common. A culture result will normally include the annotation CFU/ml which stands for "colony-forming units per milliliter", and is a measurement of the amount of bacterial cells in the sample. For example, a positive UTI might read: >100,000 CFU/ml E. coli.
You might receive this result in the form of paperwork or by notification that something has been uploaded to your medical provider’s portal.
Pros
- Can enable further testing: Because the doctor has access to a living set of the microbes present in your sample, they can test it for antibiotic susceptibility or other experimental procedures.
- Quantitative: Due to the way culturing is done, we can count the number of bacterial colonies that grow to determine how many of that specific bacteria were alive in a sample.
Cons
- Biased: Not everything can be grown in a lab, so you can miss organisms that are present and may be contributing to your symptoms.
- Slow: Culture is time-consuming and requires a lot of manpower by highly skilled professionals. Some organisms take days/weeks to grow or require special equipment to grow. Additionally, once the microbe has grown, culturing requires highly skilled professionals to actually identify which microbe is growing in the petri dish.
4. Vaginal pH test
A vaginal pH test measures the acidity or alkalinity of vaginal fluid. The normal vaginal pH range is typically between 3.8 and 4.5, which is slightly acidic. This acidity helps maintain a healthy balance of bacteria and prevent infections. A pH above this range can indicate an imbalance, often associated with infections such as bacterial vaginosis or trichomoniasis.
How it works
A vaginal pH test will simply tell you whether your vaginal discharge is acidic or alkaline. After you collect a small sample of vaginal discharge, you wipe the vaginal swab on the test area of the pH test strip. The color of the test area will start changing right away. You'll then need to match the color to the closest color block on the color chart included in the test kit.
What does a result look like?
The vaginal pH test strip is compared to a color chart provided with the test kit to determine the pH level. At-home testing kits for bacterial vaginosis at home often include pH test strips to help diagnose the condition. The colors correspond to different pH levels, ranging from acidic to alkaline. A high pH could indicate bacterial vaginosis or trichomoniasis.
Pros
- Affordable: vaginal pH test kits are generally inexpensive and available at most pharmacies or drugstores.
- Fast: taking the test is quick and results are almost immediate.
Cons
- Imprecise: a vaginal pH test only measures the pH of your discharge. The test alone can't diagnose vaginal infection, nor will it tell you which microbes are present in your vaginal microbiome.
- You'll need a follow-up: if the test results show that your vaginal pH is outside a normal range, your healthcare provider may recommend further testing to diagnose bacterial vaginosis or another vaginal infection.
DNA-based testing methods
As you may remember from high school biology, DNA is the code of life. Every single organism — including bacteria and yeast — has a unique genetic code.
DNA is made up of a long string of four base pairs (A, T, C, & G) strung together in many combinations. With DNA testing, we can read this genetic code and use it to identify which organisms are present in a sample. However, different methods look at the DNA in different ways, and there are pros and cons to each method.
Before we jump into our explainers, here are definitions for a few terms to know when thinking about DNA testing:
- Genome: The complete set of DNA in a cell that codes for everything the organism does. Genomes vary in size for example, the human genome is 700 times larger than a microbial genome like E. coli (3.2 billion vs 5 million base pairs long)
- Gene: A segment of the genome that codes for one protein. That protein usually has a very specific function within the cell. An average bacterial cell encodes about 5000 different genes.
- Sequencing: A process by which we can extract the DNA from an organism or sample and read its genetic code. Sometimes sequencing uses primers and PCR, and sometimes not. Sometimes it sequences all DNA in a sample (shotgun metagenomics), and sometimes it sequences one specific target gene (16S amplicon sequencing).
5. qPCR
PCR (polymerase chain reaction) is a method commonly used at the doctor’s office to test for microbes like Gardnerella, Candida, and Trichomoniasis in a vaginal sample. qPCR is a version of PCR that allows you to know exactly how many copies of that gene were present in the sample.
How it works
PCR is a process where a specific section of DNA is amplified to high enough concentrations that it can be detected. Short segments of DNA, called primers, are used to bind to a specific region of DNA allowing the amplification process to proceed. Primers are proprietary and can range from lab to lab which means different labs may have different accuracy and specificity.
PCR is very useful when you are trying to detect something very specific. Because it’s so targeted, it can literally find the genetic equivalent of a needle in a haystack. Actually, because it's an amplification-based process, it can detect an organism even if that bacteria makes up only 1 cell in 10,000 — so forget a needle in a haystack: it’s a needle in a whole field of haystacks.
For example, it’s perfect for identifying cancer mutations or COVID-19. But PCR isn’t going to provide any information about other organisms in the microbiome other than what it was specifically designed to look for.
What does a result look like?
A qPCR result is usually a lab report with a list of microbes reported as either negative or positive. You’ll likely receive a copy of this lab report in your doctor’s online medical portal. If your PCR test comes back positive, it might also include information about whether the result was low, intermediate, or high. Some tests will then interpret the positive/negative to guide the clinician about whether or not the positive/negative results are consistent with a yeast infection, bacterial vaginosis, sexually transmitted infection, or normal flora.
Pros
- Highly specific: It will detect what it was designed to detect even if it's in low concentrations.
- Fast: While the results aren’t provided in the clinic, they are often available within 1-3 days.
- Quantitative: If the test uses qPCR, it can provide the number of copies of a specific gene found in a sample.
Cons
- Narrow scope: It will only report on exactly what it was told to look for (i.e. Gardnerella or Candida albicans) and will not provide any information about the rest of the microbiome. Therefore it can potentially miss a microbe that's related to vaginal symptoms a patient may be experiencing.
- Hard to compare across tests: Each lab uses its own proprietary PCR primer and could have different accuracy. So one PCR primer might find one species of Gardnerella but completely miss another.
- Typically relies on the presence/absence of a microbe: Many PCR tests provide presence/absence rather than amount. If this is the case, then you don’t know if the microbe was the majority of the microbiome or just a small player.
- No context on the overall community: With PCR, you don’t get any information on the relative amounts of bacteria present or the overall bacterial community. For example, did Gardnerella make up 10% or 90% of the sample? What did the rest of the sample consist of? This fails to give both the clinician and the patient a full picture of what’s going on.
6. 16S rRNA sequencing
16S sequencing is a form of next-generation sequencing (or NGS, which is often used to refer to any sequencing technology that was invented in the 2000s). 16S sequencing uses a combination of PCR and NGS sequencing, and it’s not commonly used at doctor’s offices today.
How it works
“16S sequencing” gets its name from the 16S ribosomal sequence, a specific region of the genome that exists in all bacteria. It’s a gene required for survival, so it mutates very slowly over evolutionary time. Those slight variations can be used as a molecular clock to help us identify which bacteria is which.
16S sequencing works by looking for different mutations in the 16S gene to identify the bacteria. Unfortunately, it's not always a great source of truth. We know that certain bacteria are more likely to be detected in 16S sequencing (like Lactobacillus), while others, like Bifidobacterium, are less likely to be detected. Also, yeast/fungi don’t have a 16S gene, meaning they aren’t identified by any 16S sequencing.
Finally, 16S sequencing only sequences a small portion (less than 20%) of the 16S gene. This means there's limited information to work with for classification, which can make it hard to distinguish between different species of certain microbes.
This is a problem when you’re trying to figure out which microbe might be present alongside specific symptoms. For example, if the test you use can’t identify between good bacteria and pathogens, it can be hard to understand the full picture of what’s going on.
Also, scientists are learning that while which microbes are there is important, what they're doing (their function) is also critical. For example, many people with vaginas have a microbiome dominated by L. iners, with some experiencing extreme symptoms and others with no symptoms at all. The key to understanding the difference between those microbiomes could lie in the content of their genomes and comparing their functions. What can one strain of L. iners do that another strain cannot? And how might this help explain why one person with L.iners is experiencing symptoms while the other is not? This type of nuance can’t be detected by 16S.
What does a result look like?
Results for 16S sequencing are usually a lab report with a list of organisms followed by the representative percentage within the community (relative abundance). Most of the organisms will be reported at the genus level (e.g. Escherichia coli or Mycoplasma hominis would show up as Escherichia or Mycoplasma), with only those with enough specificity in the 16S gene reported at the species level (e.g. Lactobacillus crispatus, L. gasseri, L. iners, & L. jensenii would be separated out).
Pros
- Broad(er): Provides insight into a wider list of organisms than PCR does.
- Relatively cheap: It's much more expensive than PCR but slightly cheaper than other NGS technologies (like metagenomics, described below).
Cons
- Slow: It often takes 1-3 weeks to report results.
- Bacteria only: The 16S gene won't detect yeast. Another test must be done to determine if Candida is present, meaning you cannot compare the abundance of yeast to bacteria with 16S testing.
- Biased: Certain bacteria are more likely to be detected than others. For example, Lactobacillus is very easy to detect, but Streptococcus or Bifidobacterium are not.
- Non-specific: There's not enough information to classify everything to the species level. Only certain organisms, like Lactobacillus, can be classified to the species level, other organisms like Prevotella or Eschericia are often only reported at the genus level. Additionally, 16S provides no information about the potential function of the microbes present.
7. Shotgun metagenomics
Shotgun metagenomics is another type of NGS, but one that looks at the whole genome instead of just the 16S variable region. Metagenomic testing is not common at the doctor’s office and Evvy is the only at-home vaginal microbiome test to leverage this technology.
How it works
To do metagenomics sequencing, an enzyme is added that breaks all DNA into fragments (this is the shotgun part), and then those fragments are sequenced. This means that metagenomic sequencing will sequence random segments of DNA from bacteria, yeast, and viruses — any and all microbial DNA is sequenced.
The trick is then putting all that information together to identify what organisms make up the community. This computational step is called bioinformatics. It’s like taking 10 different jigsaw puzzles, mixing up all the pieces, then picking a piece out at random and trying to figure out which puzzle it went to. Thankfully with modern algorithms, we can fairly easily identify which bacteria are in a particular sample.
Because this method is non-targeted, we get information about all the organisms present, enabling someone to get a full picture of their microbiome. Not only is that valuable to people looking to better understand their own bodies, but it's critical for furthering our understanding of the vaginal microbiome.
With Evvy, for the first time ever, we can study all the things in the vaginal microbiome that had been previously overlooked, which will hopefully lead to better diagnostics and treatment options for all of us.
Finally, because metagenomics is untargeted, we get DNA sequences from all over the bacterial genome, not just from one gene. This allows us to more accurately classify microbes at a more specific level, and also to identify genes that might help us discover the cause of a disease or develop gene-specific diagnostics.
What does a result look like?
Results are often returned in a lab report showing the list of microbes as a percentage of the overall microbiome, called relative abundance. However, unlike 16S, almost all microbes can be classified according to the species level.
Also, as we learn more about the vaginal microbiome with tests like Evvy, you might also start to see sub-species identification (strain level research) and information on the bacterial functions (such as how they relate to symptoms) in metagenomic results.
Pros
- Most comprehensive: This type of testing will detect everything in a sample — yeast, bacteria, viruses, everything. We can detect previously overlooked organisms, and help identify important bacteria that have been ignored by science.
- Most precise: Because the test sequences the whole genome instead of just one variable region like 16S, you’re able to get the most precise information about which species are present in a sample. As the research progresses, this can start to include information on which strains are present and if any specific bacterial genes might be causing vaginal symptoms.
Cons
- Resources: Metagenomics requires additional time and money for high sequencing power and analyzing huge amounts of data.
- Turnaround time: It takes 1-2 weeks to turn around results.
- A lot of detail: Since metagenomics identifies everything present, the results can feel overwhelming. You may see organisms that you, or your doctor, are unfamiliar with. (This is why Evvy’s results experience comes with transparent information on what science knows so far, in-depth explanations, and links to relevant research.)
Why is it so hard to diagnose a vaginal infection?
Phew, that was a lot of testing methods to get through! But if we have so many ways to check on our vaginal health, why do so few people with vaginas feel like they get answers?
Even though DNA technology has progressed rapidly, the amount of research into women’s health (and specifically vaginal health) has unsurprisingly lagged — another reason to focus on closing the gender health gap.
Frustratingly, when it comes to vaginitis, most clinicians still rely on Amsel or Nugent scores to determine a diagnosis. And if and when they do order a lab test, it’s almost always a limited PCR panel. Remember, a test is only as good as what it was designed to do — and most vaginal health tests were designed to look for a limited number of organisms because of the limited research that exists on vaginal health.
Given the complexity of the vagina, our goal is that Evvy’s community and platform will encourage more clinicians and people with vaginas to demand a more holistic picture of the vaginal microbiome rather than the simplified approaches commonly used today.
Along the way, we’re laser-focused on leveraging the best technology to provide everyone with a vagina with the most comprehensive, personalized, scientifically rigorous education on their own vaginal health. That means being a part of Evvy means being a part of pushing science forward, and making health care better for all people with vaginas!